Abstract
Introduction Rituximab maintenance after frontline treatment has significantly improved progression-free survival (PFS) in follicular lymphoma (FL) and PFS and overall survival (OS) in mantle cell lymphoma (MCL). Patients with hematologic malignancies are at higher risk of morbimortality from COVID-19, particularly those with B-cell NHL treated with immunochemotherapy (ICT) including anti-CD20 MoAbs which, in addition, deeply impair the seroconversion rate after vaccination.
The aims of our study were to explore the incidence and severity of the COVID-19 pandemic in patients with FL and MCL receiving anti-CD20 maintenance infection and their seroconversion rate.
Patients and Methods We retrospectively reviewed all patients diagnosed with FL and MCL from March 2020 to March 2022, treated upfront (1L) and eligible for maintenance therapy with rituximab or other anti-CD20 MoAb in 6 tertiary centers from March 2020 to March 2022. Patients starting maintenance therapy, either starting it during the study period or already on treatment by March 2020 were included in the "maintenance” group and compared with those not receiving maintenance.
The severity of the COVID-19 infection was graded according to the need for either hospitalization or intensive unit care (ICU) care. Seroconversion rate was assessed according to the positivity of antibodies titer.
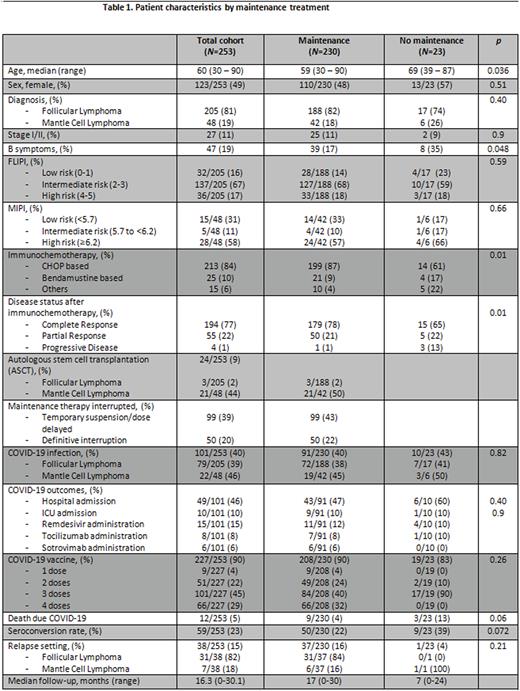
Results Out of 253 patients included in the study (205 with FL and 48 with MCL), 230 (91%) received maintenance, whereas 23 (9%) did not. The reasons for not receiving maintenance were progressive disease in 3 cases (13%) and physician's decision based on advanced age, comorbidities or COVID-related conditions in the remaining 20 patients.
Median age for the overall cohort was 60 years, but patients not receiving maintenance were older (69 vs. 59 years, p=0.036). One hundred and twenty-seven (49%) were female and 27 (11%) had limited stage disease. FLIPI was categorized as intermediate risk for most (67%) FL patients and MIPI as high-risk for most (58%) MCL. Risk categories in both diseases were well balanced between maintenance and non-maintenance.
Induction treatment was CHOP-based in 213 patients (84%), bendamustine-based in 25 (10%) and other regimens in 15 patients (6%), all of them in combination with an anti-CD20 MoAb. After induction, 194 patients (77%) attained a complete response (CR), 55 (22%) a partial response (PR) and 4 patients (1%) progressive disease (PD). Twenty-four patients (9%) received consolidation with autologous stem cell transplantation (ASCT) after 1L.
Temporary interruptions or dose delays during maintenance due to COVID-19 infection or vaccination were reported in 99 cases (39%) and definitive suspension in 50 (20%) patients. COVID-19 infection was diagnosed in 101 patients (40%), of which 49 (48.5%) were hospitalized due to severe COVID-19 infection and 10 patients (10%) of all those admitted requiring ICU care. No significant differences in either infection rate or severity were observed between maintenance and non-maintenance patients. Up to 227 patients (90%) were vaccinated against SARS-CoV2, most of them receiving ≥3 doses (72% of FL and 90% of MCL), being Spikevax™ (Moderna) being the most frequent brand used. The seroconversion rate was 23% in the overall cohort, with a trend to higher seroconversion rate in patients not receiving maintenance (39% vs. 22%).
With a median follow-up of 16.3 months, 38 patients (15%) have relapsed (31 FL and 7 MCL) and 23 (9%) patients have died due to COVID-19 pneumonia (12 patients [5%], 9/230 in the maintenance group, and 3/23 in the non-maintenance group), causes related to lymphoma (4 patients) and other causes (7 patients).
Conclusions Despite immunochemotherapy being associated with a higher risk of COVID-related complications, neither the rate nor severity of COVID-19 infection were higher in the group of maintenance with anti-CD20 MoAbs in comparison with the patients not receiving maintenance. Nevertheless, a trend to a lower seroconversion rate after vaccination was observed in the maintenance group. COVID-19 infection caused treatment delays in 40% and suspensions in 20% of patients receiving maintenance therapy. Modifications in maintenance due to COVID-19 infection could have long-term consequences on the course of these diaseases, especially in the case of MCL patients.
Disclosures
Sancho:Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria; Kern Pharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Sandoz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Eli Lilly & Company: Consultancy, Membership on an entity's Board of Directors or advisory committees; Miltenyi Biomedicine: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celltrion: Consultancy, Membership on an entity's Board of Directors or advisory committees. Gonzalez Barca:ABBVIE: Consultancy, Honoraria, Other: TRAVEL AND ACCOMODATION; KIOWA: Consultancy; ROCHE: Other: TRAVEL AND ACCOMODATION; BEIGENE: Consultancy; JANSSEN: Consultancy, Honoraria, Other: TRAVEL AND ACCOMODATION; TAKEDA: Honoraria; EUSA PHARMA: Consultancy, Honoraria; ASTRAZENECA: Honoraria. Cordoba:GenMab: Consultancy; Takeda: Consultancy; Gilead: Honoraria; Celgene: Consultancy, Honoraria; Bristol Myers: Research Funding; Pfizer: Consultancy, Speakers Bureau; Kite: Consultancy. García:Janssen, Roche, Gilead, Celgene: Consultancy; Janssen, Abbvie: Research Funding; Janssen, Roche, Gilead, Celgene, Abbvie: Other: medical meetings funding. Cabirta:Jazz: Honoraria; AstraZeneca: Honoraria; Janssen: Honoraria. Marin-Niebla:Janssen: Consultancy, Honoraria; Lilly: Consultancy, Honoraria; Kiowa Kirin: Consultancy; Takeda: Consultancy, Honoraria; Astra-Zeneca: Consultancy; Roche: Consultancy; Kite: Consultancy, Honoraria. Bosch:Roche: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Celgene/BMS: Consultancy, Honoraria; Karyospharm: Honoraria; Astra Zeneca: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Mundipharma: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Lilly: Consultancy, Honoraria; Beigene: Consultancy, Honoraria. Abrisqueta:Sandoz: Honoraria; Incyte: Consultancy; Roche: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Speakers Bureau; BMS: Consultancy, Honoraria, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal